How The Mask Works
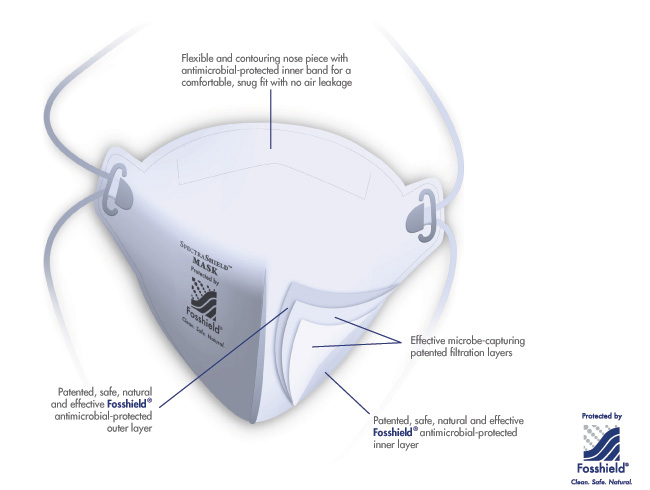
Filtration Technology
The filtration technology in the SpectraShield™ Series of antimicrobial respirator masks formally passed penetration and resistance in multiple testing at numerous independent testing laboratories in the European Union. These tests require the SpectraShield™ masks to be subject to exposure of a quantity of particulate aerosols at .3 micron in size at a specific velocity rate. Upon the exposure of the aerosols, the amount of droplets that penetrate the mask are measured. In the European Union, for the masks to be rated a FFP2 it must meet a minimum of a 97% filtration rate.
In addition to conventional testing for a disposable respirator mask, the SpectraShield™ mask was also subjected to rigorous testing for reusability in which each mask was tested for filtration performance, inhalation and exhalation minimum tolerances after the masks had been subjected to severe clogging in a dolomite dust test. Two of the SpectraShield masks passed this rigorous reusability testing to earn a classification of FFP2 RD.
The tests conducted at these various independent laboratories were conducted according to national and international testing standard for filtration and safety. All the independent testing laboratories used in testing the SpectraShield™ masks are recognized by the applicable regulatory agencies in the European Union.
Toxicology
Extensive toxicology testing has been performed by AgION regarding the silver-copper zeolite antimicrobial agent. Independent tests results indicate the antimicrobial agent to be safe and non-toxic causing no negative side affects, conditions, or consequences.


